Access curated Anaesthesia Devices procurement intelligence and solve all your questions on Anaesthesia Devices procurement outlook, cost saving opportunities in Anaesthesia Devices procurement and potential Anaesthesia Devices partners.
GET FREE SAMPLEAnaesthesia Devices is an essential part of the Medical and Surgical Equipment and Supplies category which includes all spend related to any durable machine, instrument, implement or implant used by clinicians for the diagnosis, prevention, monitoring or treatment (whether invasive or non-invasive) of medical conditions. The products may also be used for life support, modification or replacement of part(s) of anatomy, conception control and disinfection of other medical equipment.
A changing ecosystem within the Medical and Surgical Equipment and Supplies market also affects the procurement process, cost dynamics and supplier attractiveness for the buyers of Anaesthesia Devices. Over the years, regional boundaries have blurred as suppliers foray into different continents, increase their capital expenditure and spend on cross-border M&A initiatives. The deployment of geographic diversification strategies through such consolidation initiatives has enabled the large suppliers to improve their performance and expand their business footprint.The growing need for highly skilled workforce specialized in Anaesthesia Devices is driving suppliers to increase compensation for experience professionals to retain them. This is inflating their OPEX and causing increase in procurement costs.Many suppliers are expanding within the value chain to offer integrated solutions. With this new approach, suppliers can offer a wide portfolio of solutions and position themselves for large contracts that go beyond just Anaesthesia Devices. Buyers also find the benefits of volume buying, consolidation of supplier base, efficient co-ordination in case of an event and less spending on category management.
The report discusses in detail the best practices that have served well the category managers responsible for Anaesthesia Devices procurement. For example, Buyers should look to minimize the contract-related risks where unfavorable credit terms may severly penalize buyers for delayed payments or entitle the supplier to demand pre-payments or shorten the payment terms. Although these clauses are not very common in the category, suppliers may still insist on them, especially in situations where buyers' liquidity or financial health is in question. Before engaging in a contractual agreement with suppliers, buyers should focus on identifying and understanding various critical parameters along with their impacts on different procurement models. Buyers must clearly define clauses pertaining to the ownership of subcontracted services that are not part of their service providers' portfolios. This is necessary to ensure service consistency and control over sensitive data that are shared with sub-contracted firms.
Activate your free account to gain easy access to cutting edge research and insights on consumers, emerging price trends, global and regional suppliers.
Anaesthesia Devices procurement managers also need to proactively identify and mitigate potential risks that can arise in the supply chain or contracts for Anaesthesia Devices procurement. Some examples include:
For detailed insights and complete access to our report library, activate your free account!
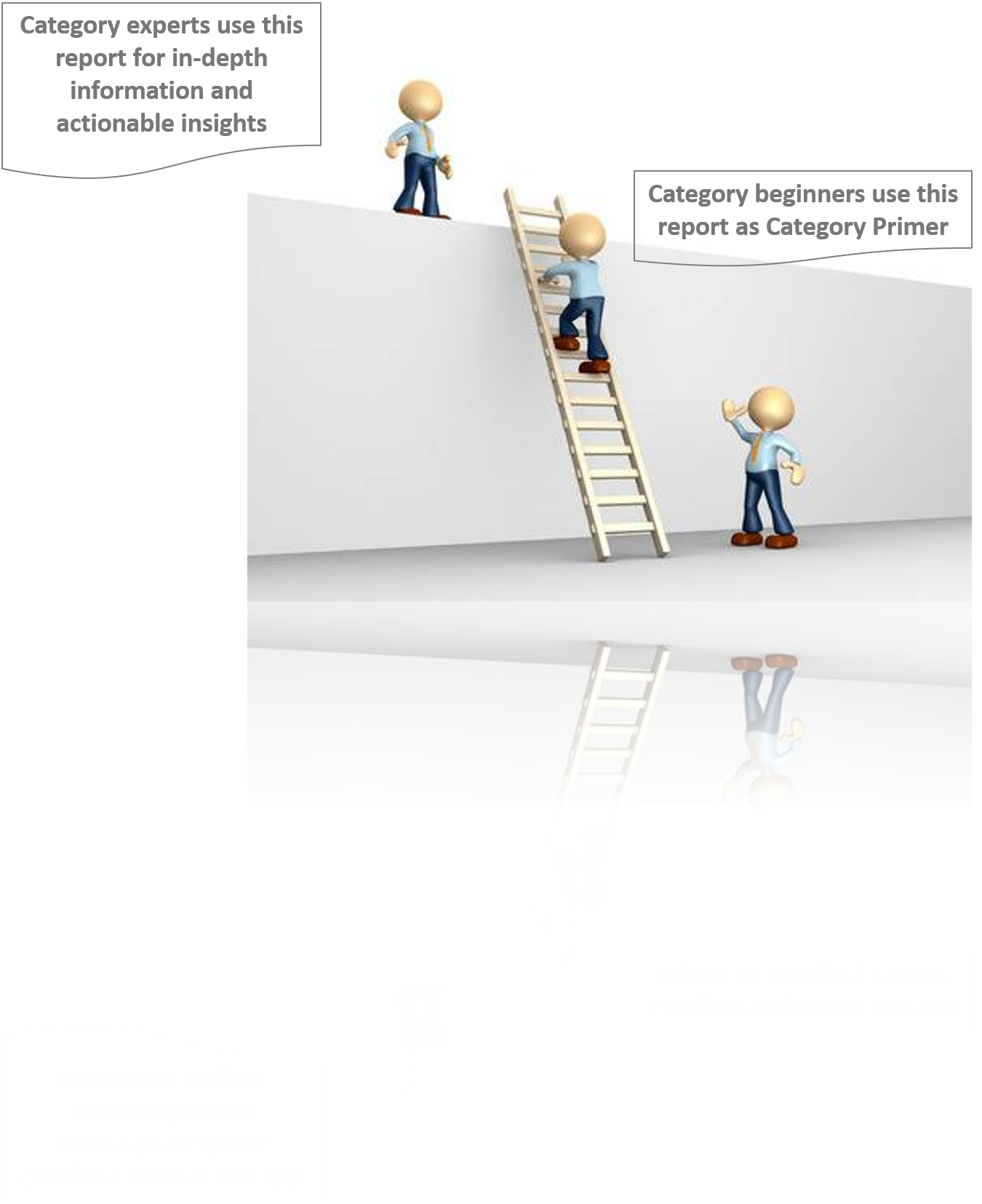
The report is intended to serve as a one-stop reference guide for Anaesthesia Devices procurement strategy and offers a perfect blend of category basics with deep-dive category data and insights. Therefore, it is ideal for category beginners looking for “Anaesthesia Devices: Procurement Report 101” as well as for category experts actively tracking the global Anaesthesia Devices procurement market.
You may have just initiated your research to design a winning Anaesthesia Devices procurement strategy, or you may be a category expert looking for strategic insights and updated data.Either ways, the report has your requirements covered.
Unlock SpendEdge's comprehensive procurement report collection with ease through our procurement platform.
Procurement decisions can prove to be costly in the absence of careful deliberation and evaluation of every available option. In fact, more than 90% of the decision makers we work with acknowledge that timely availability of up-to-date category intelligence can help them make better purchasing decisions. More than 80% of them believe that in-house category intelligence needs to be updated periodically to achieve full benefits. If you have read so far, we are quite sure you agree!!
The Anaesthesia Devices procurement report helps take more informed decisions by placing all the critical information and advice at the fingertips of a decision maker. It also specifically answers some of the key questions that we have been routinely asked during our industry outreach initiatives:
SpendEdge Insights has helped procurement professionals and sourcing teams manage multiple spend areas and achieve more than $2 billion in savings. Activate your free account today!
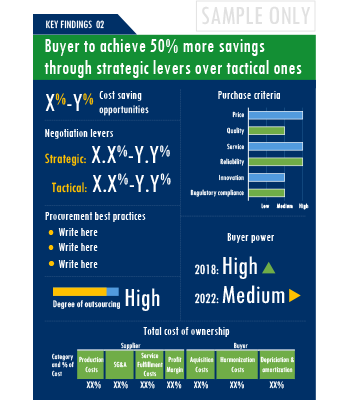
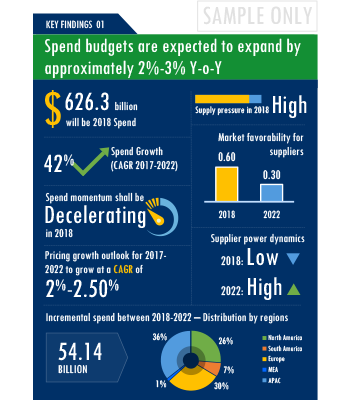
The Anaesthesia Devices market report offers a complete picture of the supply market and analyzes the category from the perspective of both buyers and suppliers. Analysis of the category trends, procurement best practices, negotiation levers and overall category management strategy advisory are interspersed with in-depth data and commentary on spend outlook, pricing ecosystem and supplier landscape drilled down to a region-level coverage.

A key highlight of this report is the in-depth outlook created on Anaesthesia Devices procurement spend and pricing trends. The report further delves deep into the aspects of cost structure, total cost of ownership and supplier margins for Anaesthesia Devices. A dedicated section to supplier profiles and evaluation helps decision makers cast a wider procurement net and identify gaps in existing relationships.

Along with specific category and supplier intelligence, the publication also includes curated insights on Anaesthesia Devices market trends, price influencers and inherent risks. These insights help the decision makers prepare for market shaping trends in advance and create alternative strategies for changes in the market conditions.

Additionally, the report also advises on the best practices and strategies to manage the Anaesthesia Devices category efficiently. Negotiation levers and opportunities are explained in detail along with quantification of their potential. Benchmark KPIs for supplier and buyer performance management are also aggregated to better organize the category objectives. Other themes of advisory include ideal procurement organization structure, enablers to achieve KPIs or category objectives and ideal SLAs to have with suppliers.






Our research is complex, but our reports are easy to digest. Quantitative analysis and exhaustive commentary is placed in an easy to read format that gives you an in-depth knowledge on the category without spending hours to figure out “what does it mean for my company?”
SpendEdge presents a detailed picture of Anaesthesia Devices procurement solutions by way of study, synthesis, and summation of data from multiple sources. The analysts have presented the various facets of the market with a particular focus on identifying the key category influencers. The data thus presented is comprehensive, reliable, and the result of extensive research, both primary and secondary.

Global Resuscitation Devices Market - Procurement Intelligence Report

Global Medical Breathing Devices Market - Procurement Intelligence Report

Global Oxygen Tents Market - Procurement Intelligence Report

Global Blow Bottle Market - Procurement Intelligence Report

Global Oxygen Mask Market - Procurement Intelligence Report

Global Anaesthesia Mask Market - Procurement Intelligence Report

Global Resuscitation Mask Market - Procurement Intelligence Report

Global Hyperbaric Chambers Market - Procurement Intelligence Report

Global Oxygen Kits Market - Procurement Intelligence Report
Access this report and our entire procurement platform | Plans starting from USD 3000/ Year Buy Now
Copyright © 2024 Infiniti Research Limited. All Rights Reserved. Privacy Notice – Terms of Use – Sales and Subscription
Cookie Policy
The Site uses cookies to record users' preferences in relation to the functionality of accessibility. We, our Affiliates, and our Vendors may store and access cookies on a device, and process personal data including unique identifiers sent by a device, to personalise content, tailor, and report on advertising and to analyse our traffic. By clicking “I’m fine with this”, you are allowing the use of these cookies. You may change your settings based on a legitimate interest at any time, by selecting “Manage Settings” on our site. Please refer to the help guide of your browser for further information on cookies, including how to disable them. Review our Privacy & Cookie Notice.