A buying guide for Clinical Trials Investigator Portal category – enriched with research on Clinical Trials Investigator Portal procurement spend patterns, Clinical Trials Investigator Portal pricing intelligence, market dynamics and top suppliers of Clinical Trials Investigator Portal.
GET FREE SAMPLEOur category grouping process defines that the overall Science and Environmental Research market includes all spend related to research of scientific, medical, technological and environmental subjects for commercial or social purposes. Dynamics of the global Clinical Trials Investigator Portal market play out within this wider arena of Science and Environmental Research market. Therefore, our coverage of the Clinical Trials Investigator Portal category also operates within this broader boundary of the overall Science and Environmental Research market.
Science and Environmental Research market around the world is undergoing rapid transitions that have a massive potential to influence the way companies strategize for Clinical Trials Investigator Portal procurement and the cost they have to incur. Service providers are increasingly investing in the adoption of automation software and advanced technologies to realize process efficiencies, broaden their service offerings and reduce time to market. This is increasing their OPEX, which will be passed on to buyers in the form of slightly higher procurement costs. There are only a handful of \"Tier 1\" vendors in the Clinical Trials Investigator Portal market and they have traditionally enjoyed a premium in their pricing. However, Tier 2 suppliers are giving them a tough competition now as they can provide similar services at a lower cost. Many tier 2 suppliers achieve this parity by finding right talent at a relatively lower cost and maintaining lean operations without any frills. New locations and vendors are emerging as cost effective suppliers of Clinical Trials Investigator Portal, offering an opportunity to widen the supplier base and gain further negotiation leverage with incumbent suppliers.
As market conditions become more dynamic and procurement practices get more sophisticated, category managers need to be cognizant of the best practices that work for their Clinical Trials Investigator Portal category procurement. The report offers a succinct analysis of Clinical Trials Investigator Portal procurement best practices. For example, Buyers can engage in pilot projects with interested service providers to assess their capabilities. This also helps buyers to assess the cost benefits and value proposition of the proposal. Buyers should engage with service providers through a rate card model. Within the rate card model, Customers are provided with rate cards that contain pre-determined prices for various activities and resources required for the project. Price is fixed based on per resource hour for different job roles. This pricing model ensures that there is optimal utilization of supplier's resources. Buyers must provide all key requirements in detail and ensure that service providers offer precise details regarding processes, systems, manpower, and expected outcome. Regular and effective communication between buyers and service providers are crucial during the contract formation phase, as well as across different phases of the project.
Activate your free account to gain easy access to cutting edge research and insights on consumers, emerging price trends, global and regional suppliers.
Clinical Trials Investigator Portal procurement managers also need to proactively identify and mitigate potential risks that can arise in the supply chain or contracts for Clinical Trials Investigator Portal procurement. Some examples include:
For detailed insights and complete access to our report library, activate your free account!
The report is intended to serve as a one-stop reference guide for Clinical Trials Investigator Portal procurement strategy and offers a perfect blend of category basics with deep-dive category data and insights. Therefore, it is ideal for category beginners looking for “Clinical Trials Investigator Portal: Procurement Report 101” as well as for category experts actively tracking the global Clinical Trials Investigator Portal procurement market.
You may have just initiated your research to design a winning Clinical Trials Investigator Portal procurement strategy, or you may be a category expert looking for strategic insights and updated data.Either ways, the report has your requirements covered.
Unlock SpendEdge's comprehensive procurement report collection with ease through our procurement platform.
Procurement decisions can prove to be costly in the absence of careful deliberation and evaluation of every available option. In fact, more than 90% of the decision makers we work with acknowledge that timely availability of up-to-date category intelligence can help them make better purchasing decisions. More than 80% of them believe that in-house category intelligence needs to be updated periodically to achieve full benefits. If you have read so far, we are quite sure you agree!!
The Clinical Trials Investigator Portal procurement report helps take more informed decisions by placing all the critical information and advice at the fingertips of a decision maker. It also specifically answers some of the key questions that we have been routinely asked during our industry outreach initiatives:
SpendEdge Insights has helped procurement professionals and sourcing teams manage multiple spend areas and achieve more than $2 billion in savings. Activate your free account today!
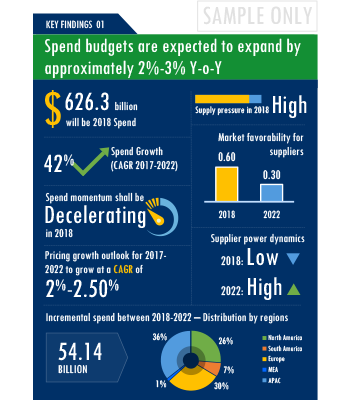
The Clinical Trials Investigator Portal market report offers a complete picture of the supply market and analyzes the category from the perspective of both buyers and suppliers. Analysis of the category trends, procurement best practices, negotiation levers and overall category management strategy advisory are interspersed with in-depth data and commentary on spend outlook, pricing ecosystem and supplier landscape drilled down to a region-level coverage.

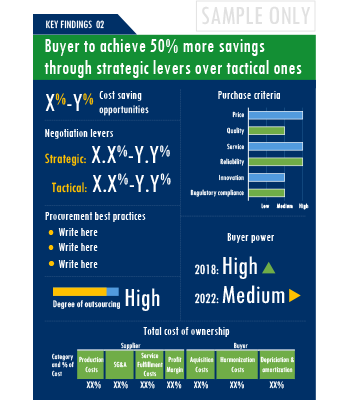
A key highlight of this report is the in-depth outlook created on Clinical Trials Investigator Portal procurement spend and pricing trends. The report further delves deep into the aspects of cost structure, total cost of ownership and supplier margins for Clinical Trials Investigator Portal. A dedicated section to supplier profiles and evaluation helps decision makers cast a wider procurement net and identify gaps in existing relationships.

Along with specific category and supplier intelligence, the publication also includes curated insights on Clinical Trials Investigator Portal market trends, price influencers and inherent risks. These insights help the decision makers prepare for market shaping trends in advance and create alternative strategies for changes in the market conditions.

Additionally, the report also advises on the best practices and strategies to manage the Clinical Trials Investigator Portal category efficiently. Negotiation levers and opportunities are explained in detail along with quantification of their potential. Benchmark KPIs for supplier and buyer performance management are also aggregated to better organize the category objectives. Other themes of advisory include ideal procurement organization structure, enablers to achieve KPIs or category objectives and ideal SLAs to have with suppliers.






Our research is complex, but our reports are easy to digest. Quantitative analysis and exhaustive commentary is placed in an easy to read format that gives you an in-depth knowledge on the category without spending hours to figure out “what does it mean for my company?”
SpendEdge presents a detailed picture of Clinical Trials Investigator Portal procurement solutions by way of study, synthesis, and summation of data from multiple sources. The analysts have presented the various facets of the market with a particular focus on identifying the key category influencers. The data thus presented is comprehensive, reliable, and the result of extensive research, both primary and secondary.

Global Outsourced Phase IV Studies Market - Procurement Intelligence Report

Global Electronic Patient-Reported Outcome (ePRO) Services Market - Procurement Intelligence Report

Global Drug Safety Market - Procurement Intelligence Report

Global Academic Research Organisations Market - Procurement Intelligence Report

Global Research Preformulation Services Market - Procurement Intelligence Report

Global Clinical Trials Investigator Portal License Agreements Market - Procurement Intelligence Report

Global Cro Medical Writing Market - Procurement Intelligence Report

Global Epidemiology Or Real World Data Services Market - Procurement Intelligence Report

Global Clinical Supply Management Market - Procurement Intelligence Report
Access this report and our entire procurement platform | Plans starting from USD 3000/ Year Buy Now
Copyright © 2025 Infiniti Research Limited. All Rights Reserved. Privacy Notice – Terms of Use – Sales and Subscription
Cookie Policy
The Site uses cookies to record users' preferences in relation to the functionality of accessibility. We, our Affiliates, and our Vendors may store and access cookies on a device, and process personal data including unique identifiers sent by a device, to personalise content, tailor, and report on advertising and to analyse our traffic. By clicking “I’m fine with this”, you are allowing the use of these cookies. You may change your settings based on a legitimate interest at any time, by selecting “Manage Settings” on our site. Please refer to the help guide of your browser for further information on cookies, including how to disable them. Review our Privacy & Cookie Notice.